Novel inhibitors of Pseudomonas aeruginosa LecB
Challenge
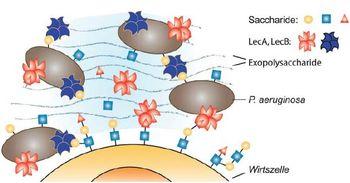
Pseudomonas aeruginosa accounts for a large number of nosocomial infections in immunocompromised hosts and in addition colonizes the lungs of patients suffering from cystic fibrosis. This opportunistic, ubiquitous gram-negative bacterium is able to switch to the biofilm mode of life, which serves as a physical barrier to survive antibiotic treatment and host immune defense. In addition P. aeruginosa develops high resistance towards antibiotics resulting in maintenance of chronic infections and high mortality of infected patients. For biofilm formation and maintenance of the biofilm architecture special lectins, LecA and LecB, are necessary, making these lectins suitable targets for inhibiting drugs acting as blockers of P. aeruginosa`s pathogenicity. As there are related lectins which are crucial for the immune system of the human host, there is a need for specific, potent P. aeruginosa lectin inhibitors that do not block the host's immune system.
Technology
The present invention relates to compounds derived from 1-deoxy fucose, which act as lectin inhibitors, especially as inhibitors of LecB, for therapeutic use in prophylaxis or treatment of infections such as nosocomial infections with P. aeruginosa and respiratory tract infections of patients suffering from cystic fibrosis. The novel selective LecB inhibitors can preferably be administered orally and bind to P. aeruginosa LecB with high affinity in the nanomolar range. Thereby, these small molecules inhibit the generation of P. aeruginosa biofilms, which are crucial for the pathogenicity of this organism, sensitising them for antibiotic treatment.
Commercial Opportunity
The invention is offered for licensing and co-development.
Development Status
Data from in vitro assays available, cell-based assays ongoing.
Patent Situation
A European priority application was filed in March 2015, international PCT-application filed on March 24, 2016 (WO2016/151066), national applications pending in EP, US, CA, JP, AU and CN.
Further Reading
Sommer et al. 2018. Glycomimetic, Orally Bioavailable LecB Inhibitors Block Biofilm Formation of Pseudomonas aeruginosa. J. Am. Chem. Soc. 2018, 140, 2537-2545.
Sommer et al. 2016. The virulence factor LecB varies in clinical isolates: consequences for ligand binding and drug design. Chem. Sci. 2016, 7, 4990-5001