Targeting host lipid homeostasis to augment anti-mycobacterial treatment
Invention Novelty
Tuberculosis (TB), caused by Mycobacterium tuberculosis (Mtb), represents one of the most prevalent infectious diseases, and is responsible for the largest fraction of infection-related casualties (before HIV). According to WHO estimates, about 10 million people contracted TB in 2019 and 1.4 million people died from the disease. The increasing emergence of multidrug-resistant (MDR) and extensively drug-resistant (XDR) TB jeopardizes TB control and undermines the goals of the WHO’s END TB initiative. In fact, more than 5% of all TB patients suffer from MDR-TB and must be treated with second-line drugs, which are more toxic and expensive than first line drug and are effective in only about 57% of all cases. This already unsatisfactory situation is further complicated by the emergence of virtually untreatable XDR-TB. Hence, novel, and effective drugs are urgently needed, and the innovation provides a valuable host-directed strategy to improve MDR-/XDR-TB therapy.
Value Proposition
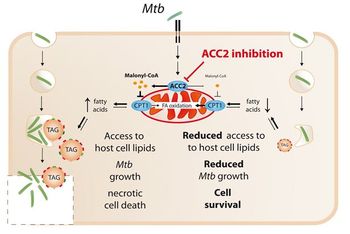
The innovation relates to a novel, host-directed approach against MDR-/XDR-TB that is based on inhibition of lipogenesis in the host. Inhibition of the key enzyme Acc2 disrupts the supply of lipids to Mtb, slowing its growth and making the bug more susceptible for conventional anti-mycobacterial therapies. Suitable Acc2 inhibitors are in development for metabolic diseases and known anti-TB drugs exert their activity at otherwise subinhibitory concentrations.
Technology Description
Investigating foamy macrophages within the lungs of TB patients and experimentally infected mice, it was found that Mtb promotes an accumulation of neutral lipids in the host via WNT6-induced expression of key lipid metabolic enzymes including acetyl-CoA carboxylase-2 (Acc2). These lipids are exploited by intracellular Mtb as predominant carbon source, and accordingly, the host`s lipogenesis provides a novel target for a host-directed TB therapy. Inhibitors of lipogenesis in general and Acc2 in particular, have been extensively studied in the past by others for the treatment of metabolic disorders, like obesity, NASH and hepatic steatosis etc., and can now be re-purposed for boosting TB therapy.
Commercial Opportunity
The technology is available for in-licensing and/or co-development. Regarding the latter, the inventing institution (FZB) can contribute extensive scientific expertise in TB research, including national and WHO supranational Mtb reference center, established mouse models with human TB pathology, and a clinical study center.
Development Status
The technology has been successfully tested e.g., in human macrophages using several commercially available Acc2 inhibitors. The tests revealed a significant, concentration-dependent inhibition of Mtb growth, although the Acc2 inhibitors did not directly affect Mtb and were no toxic towards human macrophages. Tests in established animal models showed that inhibitors of human Acc2 inhibit Mtb growth also in vivo, significantly improving standard TB therapy.
Patent Situation
The technology is protected by international PCT application WO2018/007430, which has been granted in the EU, and is pending in the US.
Further Reading
Brandenburg et al. (2019) WNT6-ACC2-induced accumulation of triacylglycerol rich lipid droplets is exploited by M. tuberculosis
. bioRxiv: doi.org/10.1101.2020.06.26.
Brandenburg et al. (2021) WNT6/ACC2-induced storage of triacylglycerols in macrophages is exploited by Mycobacterium tuberculosis. J. Clin. Invest. 131:e141833.