Anti-inflammatory, LPS-neutralizing and anti-bacterial agent against sepsis and other severe bacterial infections
Challenge
Sepsis represents one of the leading causes of death in intensive-care units worldwide, with mortality rates ranging from 20% for sepsis to over 60% for septic shock. Treatment of sepsis poses an enormous burden for the health care systems with annual costs of USD 17 bn p.a. in the US alone. Existing therapies aim at killing the bacteria or modulating the immune response. Yet, these strategies do not address the major underlying trigger for sepsis, i.e. release of pathogen-associated molecular patterns (PAMPs; i.e. endotoxins) and damage-associated molecular patterns (DAMPs; e.g. heparan sulfate). Thus, in spite of recently improved diagnostic and therapeutic options, there is a major medical need for new and efficacious drugs, which do not only kill the bacteria, but also neutralize both, PAMPs and DAMPs.
Technology
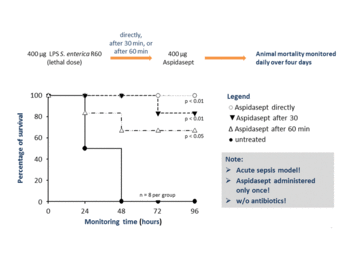
Based on comprehensive biophysical studies, a novel class of peptide-based antiinfectives has been developed against sepsis and other severe infections. Lead compound Aspidasept possesses potent antibacterial and antiviral activities, and importantly is capable to efficiently bind and neutralize PAMPs (derived from both Gram-negative and Gram positive bacteria) and DAMPs, this way preventing the overshooting inflammation reactions. A variety of in vitro and in vivo data are indicative of the compound's potent anti-inflammatory and PAMP/DAMP-neutralizing activity, without compromising the host's immune response. Protection in animal models has been demonstrated e.g. for endotoxemia and cecal ligation/punctures (CLP). Induced inflammation in lung sections could be completely inhibited, and even delayed addition of Aspidasept decreases inflammation and increases survival.
Commercial Opportunity
Aspidasept and related peptides are offered for in-licensing and/or co-development for the treatment of sepsis and/or bacterial and viral infections.
Development Status
- Given their physicochemical properties, the novel antimicrobial polypeptides possess broad activity against bacteria and viruses. Various in vitro and in vivo studies revealed potent activity against MRSA and synergistic properties with other antibiotics.
- The novel polypeptides efficiently neutralize PAMPs in vivo, reducing mortality as good or better as the accepted gold standard polymyxin B.
- Protection in animal models has been demonstrated e.g. for endotoxemia and cecal ligation/punctures (CLP). Induced inflammation in lung sections could be completely inhibited, and delayed addition of Aspidasept decreases inflammation and increases survival.
- For lead compound Aspidasept, a 14-day repeated dose toxicity study in rats has been completed. The NOAEL was determined, revealing that the planned clinical dose will be 500-times lower.
- Based on animal data, Aspidasept possesses a sufficient stability when given as permanent infusion, being degraded within 2 h, this way preventing undesired accumulation, induction of resistance and immunological/allergic reactions.
- A protocol for GMP production has been established.
- Preclinical development of Aspidasept is well advanced. Further studies are under way, in preparation and/or planned.
Patent Situation
Based on WO 2009/124721 (priority claimed: 04/2008), patents have been granted in US, JP and EP.
A second patent applications has been recently filed (WO 2017/140770; patent pending in US, JP and EP), covering e.g. the synergistic action in combination with standard-of-care antibiotics.
Further Reading
Heinbockel et al. (2013) Preclinical investigations reveal the broad-spectrum neutralizing activity of peptide Pep19-2.5 on bacterial pathogenicity factors. Antimicrob Agents Chemother 57:1480-1487
Schuerholz et al. (2013) The anti-inflammatory effect of the synthetic antimicrobial peptide 19-2.5 in a murine sepsis model: a prospective randomized study. Crit Care 17:R3
Martin et al. (2015) Peptide 19-2.5 inhibits heparan sulfate-triggered Inflammation in murine cardiomyocytes stimulated with human sepsis serum. PlosOne 10:e0127583
Martinez de Tejada et al (2015) Lipoproteins/peptides are sepsis-inducing toxins from bacteria that can be neutralized by synthetic anti-endotoxin peptides. Sci Rep 22: 14292
Martin et al. (2016) The synthetic antimicrobial peptide P19-2.5 attenuates septic cardiomyopathy and prevents down-regulation of SERCA2 in polymicrobial sepsis. Sci Rep 6:37277
Yamada et al. (2017) Novel synthetic, host-defense peptide protects against organ injury/dysfunction in a rat-model of severe hemorrhagic shock. Ann Surg epub
Bárcena-Varela et al. (2017) Coupling killing to neutralization: combined therapy with ceftriaxone/Pep19-2.5 counteracts sepsis in rabbits. Exp Mol Med 40: e345