LS-COAT – an exceptionally efficient tumor vaccination approach
Keywords
Prime boost vaccination approach, tumor vaccination approach, antigen-specific vaccination protocol
Invention Novelty
The administration of two well-defined compositions in liposomal vesicles with a prime-boost vaccination approach elicits a very efficient T-cell response against tumor cells, but can also be used for bacterial and viral infections. Therefore, the novel technology comprises an impressively effective and antigen-specific vaccination protocol, referred to as LS-COAT.
Value Proposition
The exploration of new strategies against tumors is a major challenge of current research. In this respect, several studies could verify the general effectiveness of immunotherapeutic approaches. Based on these promising research results, immunotherapy is considered to be a key for directed and efficient eradication of tumors. However, although enormous efforts have been spent on the development of new vaccination protocols, none of the novel strategies could reveal a sufficient efficacy against cancer. Thus, there is still a strong need for the development of new and efficient immunotherapeutic strategies to fight cancer.
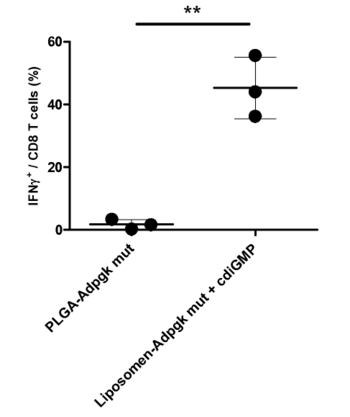
Technology Description
The newly developed technology comprises an impressively effective and antigen-specific vaccination protocol, referred to as LS-COAT. The separate administration of two well-defined compositions in liposomal vesicles with a prime-boost vaccination approach elicits a very efficient and specific T-cell response within comparatively short time. Although the novel strategy is preferably directed against tumor cells, the identified combination of compounds may also be used to raise T-cell immunity against intracellular bacterial or viral infections. Thus, the new technology gives rise to an excellent multifunctional tool for the immunotherapeutic treatment of diverse clinical indications with a particular focus on cancer.
Commercial Opportunity
In-licensing or collaboration for further development is possible.
Development Status
In vivo studies in mice were performed to validate proof of principle.
Patent Situation
European Patent with priority of 2017 has been granted in 2021 (EP3672625 B1; national validation in DE, CH, FR and GB). US patent application (US 2021/0077623A1) is pending.
Further Reading
Nimanong S, Ostroumov D, Wingerath J, Knocke S, Woller N, Gürlevik E, Falk CS, Manns MP, Kühnel F, Wirth TC. 2017. CD40 Signaling Drives Potent Cellular Immune Responses in Heterologous Cancer Vaccinations. Cancer Res. 77(8):1918-1926.