New viral packaging cell line
Keywords
Packaging,cell line,gene therapy,retroviruses,virus
Challenge
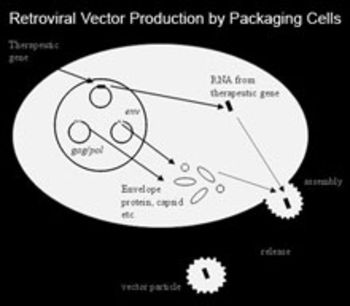
The high potential of the use of gene therapy to cure and slow down a wide spread scope of diseases has been shown in recent clinical trials. But the efficient introduction of therapeutic genes into human cells still remains the main problem. One established technique for gene transfer uses viral vectors, frequently retroviral vectors as they are robust, can be easily produced in high titers and efficiently infect a broad spectrum of cells. However, the production technology for retroviral vectors is still in early state. Inherent instability of infectious viral particles, the difficulty to produce large amounts of viruses as well as the broad host range of currently available viral envelopes and the sensitivity to human complement attack impede successful gene therapy. Presently recombinant retroviruses transducing a retroviral vector encoding for the therapeutic gene are produced after infection of the vector into packaging cell lines stably expressing necessary viral proteins (gag/pol and env). The construction of packaging cells is a tedious procedure including very time-consuming screenings. To facilitate this screening mostly therapeutic viruses carry selection or marker genes, which can cause severe problems in therapeutical settings like immune response or transduction of marker genes that turn out to be not biologically inert as they were expected to be. Therefore new processes allowing the production of large numbers of stable viruses containing no additional coding sequence besides the therapeutic gene are needed.
Technology
The invention provides new methods for the production of virus producing cell lines. With the first step a master producer cell line is generated once providing a tagged cell line selected for optimized expression of various expression cassettes. With the second step the master producer cell line is targeted with the nucleotide acid molecule of interest. Due to the predicted integration site, the titer, the cultivation conditions and the safety record are not affected. This allows standardised easy production of virus which may be used in gene therapy.
Commercial Opportunity
The technology is offered for in-licensing or co-development.
Patent Situation
European patent granted in 2010 (EP1841878B1).
Further Reading
Coroadinha et al. The use of recombinase mediated cassette exchange in retroviral vector producer cell lines: predictability and efficiency by transgene exchange. J Biotechnol. 2006 Jul 13;124(2):457-68.