Novel method for serum-free generation of respiratory epithelial cells
Challenge
A number of lung diseases such as cystic fibrosis cannot be treated adequately with conventional drugs. Since in many cases these diseases are of genetic origin, patients affected are candidates for gene therapy. With the recent advances in stem cell and iPS cell research future therapeutic options may be based on the generation of autologous stem cells from the patient’s tissue. This novel approach will include repair of the gene defect and differentiation of modified stem cells into lung tissue which will later on be transplanted into the patient’s lung. Due to a lack of protocols for respiratory differentiation of stem cells under well-defined culturing conditions, new methods are desperately needed.
Technology
Using this new protocol, stem cells can be transformed into respiratory cells by applying a combination of media additives which are key factors for respiratory differentiation. Notably, combining these factors leads to cell differentiation without the use of serum. The technology thus will facilitate the generation of respiratory epithelial cells under defined culturing conditions, which is critical for production of cell-based implants of low immunogenicity under GMP standards. Alternatively, the method can also be used to produce cells for ADME/Tox testing.
Commercial Opportunity
In-licensing or collaboration is possible.
Development Status
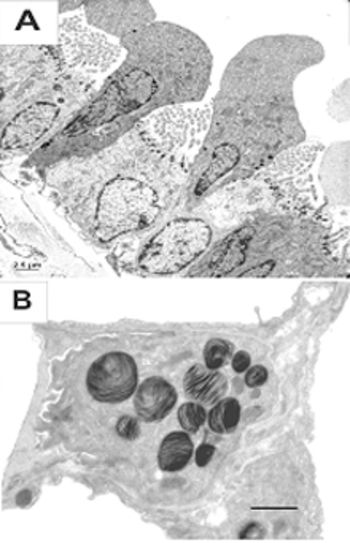
Murine embryonic stem (ES) cells and iPS cells were successfully transformed into respiratory epithelial cells characterized by surfactant protein and CCSP expression. The technology is currently optimized for human ES and iPS cells.
Patent Situation
Patent applications with priority of 2011 in USA (US 9,765,303), Europe (EP 2670841 - national validation in CH, DE, FR and GB) and Australia (AU 2012213402) have been granted.