Biodynamers enhance drug delivery
Keywords
gene therapy,transfection reagents,drug delivery system,biodynamers,dynaplex,protein delivery,vaccine,mRNA delivery
Invention Novelty
Drug delivery of anionic molecules such as nucleic acids or anionic proteins is enhanced using new cationic biodynamers. Delivery of cationic proteins can be improved with anionic biodynamers, respectively.
Value Proposition
Safe and efficient delivery of nucleotides e.g. mRNA is essential for gene therapy approaches but also for vaccines based on nucleic acids. Appropriate transfection tools should not only effectively deliver nucleic acids into the cytoplasm, protecting them from nucleases. In addition, toxicity, immunogenicity, and damage to the target cells by transfection reagents should be avoided. The most widely used polymeric transfection agent polyethyleneimine (PEI) is characterized by a high transfection efficiency but also a high cytotoxicity. The biodynamers described here show a significant increase in the number of living transfected cells compared to PEI and thus represent a new and innovative method for gene and drug delivery. Furthermore, demonstrated transfection with biodynamers was up to three-fold higher than a lipid-based commercially available transfection agent for a research tool.
Biodynamers can be used alternatively as delivery tool for proteins, anionic and cationic, depending on the charge of the amino acids used for biodynamer composition.
Technology Description
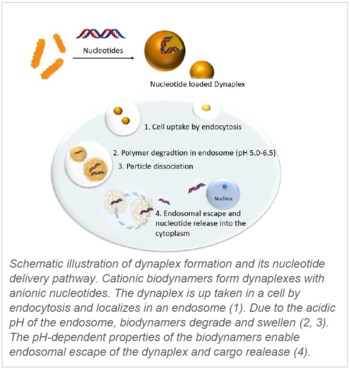
Biodynamers comprise a novel polymer system that combines the advantages of biopolymers (biocompatibility, biodegradability, biofunctionality) with dynamic covalent chemistry (reversible under certain conditions such as pH and temperature). Due to the acylhydrazone bond formation between carbazole monomers and amino acid-derived monomers (derivatives of basic amino acids e.g. Lys, His, Arg) cationic biodynamers are formed. The polymerization (formation of hydrazone bond) is reversible under acid conditions and non-reversible at neutral pH. The combination of polymerized cationic biodynamers with nucleic acids or anionic proteins leads to the formation of nanocomplex (so-called dynaplexes) by electrostatic interactions. To avoid dissociation of dynaplexes (DP) under high salt concentration cross-linking using glutaraldehyde is possible.
Commercial Opportunity
Biodynamers represent an attractive alternative to PEI and other transfection reagents due to reduced toxicity and improved transfection efficacy. The biodynamer technology can be licensed for distinct indications or defined types of molecules or for broad applications like transfection kits.
Development Status
The advantages of biodynamers were validated in in vitro studies using Lys-DPs as a gene delivery carrier. Complexes of nucleotides with Lys-DPs (crosslinked), PEI and poly-L-lysine, respectively, were compared in transfection assays. A remarkably reduced toxicity (~100 times) was observed for the Lys-DPs. When considering both transfection efficiency and toxicity, crosslinked Lys-DPs showed an improved number of living transfected cells by a factor 4 compared to PEI.
Patent Situation
A European Patent Application was filed in June 2020. Regional / national Phases in EP and US based on PCT-application were initiated in November 2022, respectively.
Further Reading
Lee, S., Nasr, S., Rasheed, S., Liu, Y., Hartwig, O., Kaya, C., Boese, A., Koch, M., Herrmann, J., Müller, R., Loretz, B., Buhler, E. Hirsch, A. K. H., Lehr, C.-M. (2023). Proteoid biodynamers for safe mRNA transfection via pH-responsive nanorods enabling endosomal escape. J Control Release, 353, 915-929. doi:org/10.1016/j.jconrel.2022.12.018
Liu, Y., Lehn, J.-M., & Hirsch, A. K. H. (2017). Molecular Biodynamers: Dynamic Covalent Analogues of Biopolymers. Accounts of
Chemical Research, 50(2), 376–386. doi:10.1021/acs.accounts.6b00594
Liu, Y., Stuart, M. C. A., Buhler, E., Lehn, J.-M., & Hirsch, A. K. H. (2016). Proteoid Dynamers with Tunable Properties.
Advanced Functional Materials, 26(34), 6297–6305. doi:10.1002/adfm.201601612