Genetically engineered human 3D brain tumor models for disease modeling and compound evaluation
Challenge
Brain tumors are among the most lethal and devastating cancers. Their study is limited by genetic heterogeneity and the incompleteness of available laboratory models. Thus, the etiology of most brain tumors is not well understood. Cell lines derived from human brain tumors are suitable for the characterization of genetic aberrations and to study gene functions. However, they cannot model effectively the key aspects of tumorigenesis, including microenvironment contribution, invasiveness, angiogenesis or inflammatory responses. In vivo models such as carcinogen-induced or genetically engineered rodent models and xenograft models provide a more accurate experimental system, as they can mimic tumor development within a mammalian organism. Nevertheless, differences in phenotypic diversity of genetic disorders between species have to be taken into account resulting in differences in tumor development and drug response between human and rodents. In this context human 3D organoid culture models offer an innovative opportunity for human disease modeling and therapy development and complement existing tools.
Technology
Human organoid tumor models have been generated by introducing oncogenic mutations during cerebral organoid formation via transposon- and CRISPR-Cas9-mediated mutagenesis. Based on clinically relevant mutations the technology has been applied to generate central nervous system primitive neuroectodermal tumor (CNS-PNET)-like and glioblastoma (GBM)-like disease models.
To obtain tumor tissue in conjunction with normal tissue only a portion of cells was transfected at the stage of embryonic body formation and visualized by GFP co-transfection. Further growth and differentiation gives rise to disease models designated as neoplastic cerebral organoids (neoCORs). These proprietary models allow to recapitulate tumorigenesis in culture over months. To verify that brain tumor-like organoids resemble distinct brain tumor subtypes transcriptome analysis, gene clustering and pathway analysis has been performed. Moreover, it has been shown that they retain viability and subtype identity after engraftment in the renal subcapsular region of nude mice. Depended on their mutagenic nature xenografts showed proliferation, and for GBMs also tumor invasion into neighboring tissue 6 weeks after implantation.
Thus, neoCORs establish valuable complements to existing preclinical models for studying genetic forms of brain carcinogenesis in vitro. They constitute novel validation and screening tools for exploratory drug discovery approaches as well as for the development of patient stratification strategies and novel therapies.
Commercial Opportunity
The technology is available for in-licensing or further co-development.
Development Status
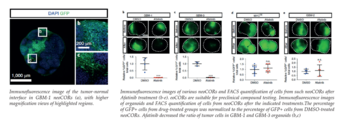
Proof of concept has been demonstrated for drug efficacy studies using the EGFR-inhibitor Afatinib, a lung cancer drug which is currently in clinical trials for treatment for glioblastoma. Different neoCORs (GBMs of subtype 1-3 and MYC overexpressing organoids) have been treated for 40 days and a significantly reduced number of tumor cells could be observed for two of the GBM subtypes, which specifically overexpress EGFR. In contrast, no effect was observed for GBM-subtype-2 organoids or in cells with strong MYC overexpression.
Patent Situation
A European patent application has been filed in 2018 followed by a PCT application (WO2019/048689). It claims the generation of human 3D tumor models and their use in studying disease phenotypes and progression, especially for neoplastic cerebral organoids (neoCORs). Moreover, it claims kit components for the generation of such organoids and their use for compound screening applications.
Further Reading
Bian et al. (2018). Genetically engineered cerebral organoids model brain tumor formation. Nature Methods 15, 631-637. https://doi.org/10.1038/s41592-018-0070-7.