Novel CAR-T against CXCR5 for treatment of non-Hodgkin lymphoma
Challenge
Chimeric Antigen Receptor T-Cells (CAR-T) have emerged as breakthrough therapeutics for the treatment of hematological malignancies with a potential for long-term remission. The vast majority of CAR-T in development target CD19, like the up to now two approved cell therapeutics Kymriah and Yescarta. Both show impressive results in B cell non-Hodgkins Lymphoma (B-NHL) which represent a heterogeneous group of blood cancers and belong to the most common cancers. The incidence in Europe and US is around 20 cases per 100.000 adults (2017) and this number will increase steadily in the future since mostly elderly people are affected. In this patient group co-morbidities exist frequently, precluding burdensome chemotherapies or allogeneic bone marrow transplantations. Furthermore, data from recent clinical trials with CD19-CAR-T show that up to 25% of patients experienced relapse due to loss of the CD19 antigen. For this group of patients, the remaining treatment options are rather low. CD19 is expressed early in B cell development and besides malignant B cells, CD19-CAR-T deplete also immature B cells. Hence, there is a huge need for CAR-T targeting alternative and more restricted antigens for the treatment of B-NHL.
Technology
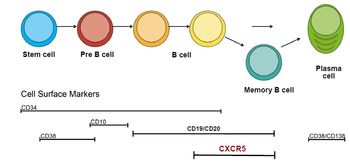
Novel CAR’s against CXC chemokine receptor type 5 (CXCR5) were developed with promising characteristics for the treatment of B-NHL. CXCR5 is an interesting target for CAR-T since expression is restricted to mature B cells and their malignant counterparts such as mature B-NHL entities and a small subset of T cells (Tfh cells). Unlike CD19, which is expressed on all B lineage cells, CXCR5 is absent on immature B cells and on plasma cells. Accordingly, B cell precursor cells are not affected by CAR-T targeting CXCR5. Despite these interesting findings, no functional CAR directed against CXCR5 has been reported so far to our knowledge. Constructs basing on a proprietary humanized CXCR5-binding domain and different signaling domains were developed, optimized and characterized in-vitro and in-vivo. Lead candidates show efficient killing of a variety of mature B-NHL cell lines, while there is no reactivity against cell lines representing B cell precursor leukemia or healthy tissue. Moreover, the lead CAR shows anti-tumor activity in-vivo in a xenotransplantation model of NSG mice engrafted with a mantle cell lymphoma cell line. Tumor cells were efficiently eradicated and regrowth was controlled over longer time. Taken together, the available CXCR5-CAR provide an attractive alternative to existing CAR-T therapies for B-NHL treatment with a potentially improved safety profile and better specificity.
Commercial Opportunity
Available for licensing and co-development
Development Status
Preclinical, in-vivo
Patent Situation
PCT patent application WO2019038368 pending, priority of 23.8.2017