Vinpocetine inhibiting PDE1, a target for disease modifying therapy of synucleinopathies like multiple system atrophy (MSA)
Challenge
Aggregates of α-synuclein (α-syn) are characteristic of synucleinopathies, a group of neurodegenerative disorders including Parkinson’s disease (PD), Lewy body dementia, and multiple system atrophy (MSA). There is currently no known therapy with proven efficacy to slow or halt the progression of synucleinopathies. Therefore, the development of new disease-modifying therapeutic strategies is of utmost importance. Cyclic nucleotide levels implicated in the control of processes including motor movement, memory and emotion are determined by the balance between the adenylyl and guanylyl cyclases that catalyze their formulation and the phosphodiesterases (PDEs) that catalyze their hydrolysis and inactivation. The PDE superfamily is comprised of several families; one of these is the PDE-1 family, which is discussed to exert a neuroprotective role.
Technology
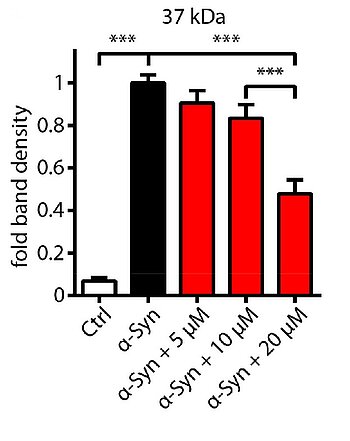
Researchers at the DZNE developed a model in which overexpression of wild-type α-syn in dopaminergic LUHMES neurons leads to 50% cell death within 6 days. Screening of 1,600 FDA-approved drugs revealed dipyridamole, an unspecific inhibitor of PDEs, to be highly protective against α-syn induced cell death. Since dipyridamole does not pass the blood-brain barrier, it does not qualify as a compound for a treatment of synucleinopathies. Further investigation of inhibitors of specific PDE isoforms resulted in the identification of the inhibitor of PDE1, vinpocetine, to protect against α-syn induced neurodegeneration. Vinpocetine is blood-brain-barrier permeable. Additional experiments suggested that the protective effect after inhibition depended mainly on cGMP, and RNA interference confirmed PDE-1A (and to a smaller extent PDE-1C) as molecular targets accounting for protective efficacy. α-syn induced nigral neurodegeneration in mice could be prevented by systemic application of vinpocetine showing that this specific PDE-1 inhibitor passed the blood-brain-barrier and protected dopaminergic neurons from α-syn toxicity also in vivo.
Commercial Opportunity
Compounds, comprising vinpocetine, inhibiting PDE-1A and -1C have been patent-protected for use in the treatment of synucleinopathies. The technology should be further developed to obtain a clinical compound. Effects of PDE-1A inhibition make this technology an exciting and novel approach for the treatment of MSA and other synucleinopathies.
The technology is open for licensing, further co-development is highly welcomed.
Development Status
A randomized, double-blind, placebo-controlled multicenter phase IIb trial to assess the effect of vinpocetine on disease progression in MSA has been applied in January 2024.
Patent Situation
Patents have been granted in US (US10918628) and EP (EP3525788, EP4112053) with priority of Oct. 11, 2016.
Further Reading
Höllerhage et al. (2017), Sci Rep. 7:11469