PADFEX- A device for direct pathogen detection in filtered expirates
Keywords
respiratory tract pathogens,microbiological diagnostic,pathogen detection,filtered expirate
Invention Novelty
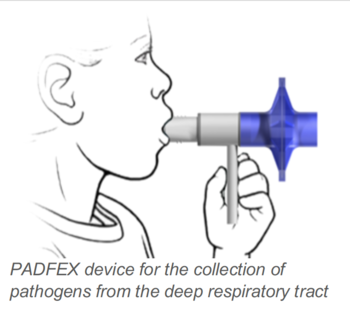
The invention provides a device for non-invasive, safe, and easy-to-use collection of pathogens from the lower respiratory tract. The PADFEX (pathogen detection in filtered expirate) device can be used by medical personnel but is also applicable as a selfsampling device.
Value Proposition
In the SARS-CoV-2 pandemic detection of pathogens in the lower respiratory tract is one of the key factors to interrupt chain of infection. Even in non-pandemic years 400,000-600,000 patients suffer from community acquired pneumonia (CAP), with 50,000 associated deaths worldwide. Simplified and rapid microbiological diagnostics can help to increase the therapeutic success of CAP treatment. Currently, diagnostics of pathogens causing respiratory tract infections uses different sampling methods such as invasive bronchoalveolar lavage (BAL), nasal-throat swabs, and sputum collection. However, while acquisition of these samples generally requires medical personnel, PADFEX is used in a non-invasive way applicable by medical personnel as well as consumers. PADFEX immobilizes aerosolized infective particles allowing detection of acute infections or to monitor epidemiological or clinical studies.
Technology Description
PADFEX consists of a novel pathogen collecting unit, mouthpiece, handhold, and filter units that prevent respiration of the exhaled breath as well as contamination of the surrounding air. After exhalation, the expirate passes the pathogen collecting unit where pathogens adhere. Mouthpiece and filter are removed and the pathogen collecting unit is contained to prevent contaminations. Finally, the pathogen collecting unit is analyzed using standard microbiological assays.
Commercial Opportunity
The technology is available for in-licensing and co-development.
Development Status
A PADFEX prototype was designed, constructed, and produced in a small series. Next to in vitro- validation studies using viral and bacterial aerosols, a first feasibility study with patients suffering from respiratory symptoms was performed providing the proof of principle.
Patent Situation
A European Patent Application was filed in December 2020 (EP20213820.2). Regional / national Phases in EP and US based on PCT-application were initiated in June 2023.