Phytosphingosine Derivatives as Adjuvants in Immune Stimulation
Keywords
Adjuvant, immune stimulation, alpha-galactosylceramid, phytosphingosine derivatives, iNKTs, vaccine, vaccination
Invention Novelty
Improved immune stimulation by novel phytosphingosine derivatives used as mucosal or parenteral adjuvants.
Value Proposition
Adjuvants are used in several vaccines to create a stronger immune response after vaccination. Currently, only a few adjuvants are approved for the use in human vaccines, such as aluminium salts, oil-in-water emulsions, and monophosphoryl-lipid A (MPL) or other TLR ligands. Since all of them show specific limitations, new and more effective adjuvants are needed to develop vaccines with improved immunogenicity. The new phytosphingosine derivatives are modifications of the immune stimulator alpha-galactosylceramide (α-GalCer; KRN 7000) that triggers different mechanism of the immune system leading to improved immunostimulatory effects. By stimulating the secretion of cytokines, the newly developed adjuvants promote cell-mediated immunity, thus improving the killing of intracellular pathogens and tumour cells, respectively, or suppressing autoreactive immune cells in autoimmune diseases. Among the novel phytosphingosine derivatives are cationic as well as anionic compounds that can be used, e.g., in liposomal formulations or viruslike particles.
Technology Description
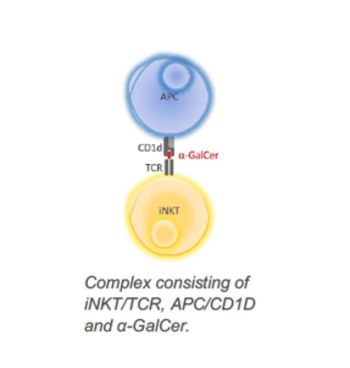
α-GalCer binds to the CD1d surface protein of antigen presenting cells (APC) and in a second step forms a larger complex with T cell receptors (TCR) of invariant natural killer T (iNKT) shown in Figure 1, resulting in activation of iNKT cells. Consequently, cytokine Th1 and Th2 are released and cells of the adaptive (antigen-specific T cells and B cells) and innate immune response (NK cells) are stimulated. The novel phytosphingosine derivatives elicit significantly improved properties in immune cell activation compared to α-GalCer. They induce proliferation of antigen specific CD19+ B cells, CD4+ B cells, and CD8+ B cells. In addition, CD4+ T cells and CD8+ T cells are stimulated.
Commercial Opportunity
The technology is available for in-licensing and co-development.
Development Status
Improved immunostimulatory effects were shown in in vitro studies using ovalbumin as antigen. Experiments on in vivo immunisation investigating Phytosphingosine derivatives as mucosal or parenteral adjuvants are ongoing.
Patent Situation
A European application was filed in June 2020. Regional / national Phases in EP and US based on PCT-application were initiated in November 2022.
Further Reading
Yanira Méndez et al., Diversification of a Novel α-Galactosyl Ceramide Hotspot Boosts the Adjuvant Properties in Parenteral and Mucosal Vaccines. Angew. Chem. Int. Ed. 2023, e202310983
Yingting Zhang et al., α-GalCer and iNKT Cell-Based Cancer Immunotherapy: Realizing the Therapeutic Potentials. Front Immunol. 2019; 10: 1126.