Flow cytometric basophil activation test for automated, high-throughput allergy diagnostics
Keywords
basophil activation, flow cytometry, gating strategy, automated analysis, allergic vs. sensitized vs. non-allergic, sample stability, IVD
Invention Novelty
Allergies have become the most common chronic disease in Western countries. Although symptomatic treatments may exist, many patients are not diagnosed correctly, as common skin prick and serological tests are only proving the presence of IgEs, but not their clinical relevance. Because IgEs alone are not a reliable marker for detecting allergies, many patients must undergo time-consuming, lengthy, and potentially life-threatening provocation tests (the gold standard). The invention provides an innovative and safe basophil activation test (BAT) that combines exceptionally performance with simplified and fully automated processing.
Value Proposition
The innovation makes superior BAT-based allergy testing for the first time accessible for cost-effective and automated routine diagnostics. Thanks to the use of three specific markers, optimal fluorophores and a sophisticated gating strategy, the BAT shows unprecedented performance and can be implemented into a fully automated workflow. Along with exceptional robustness and largely time-independent sample handling, this provides for a unique and inexpensive test for high-throughput allergy diagnostic in e.g., medical care units and hospitals, which can be even performed with blood samples send from e.g., resident physicians.
Technology Description
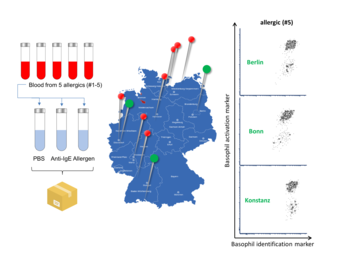
BAT mimics an allergic reaction in vitro. For this purpose, a blood sample is incubated with a potential allergen, which finds a matching, basophil coupled IgE only in case of an existing allergy. Only the IgE/allergen complex mediates cross-linking with the IgE receptor, resulting in the release of histamine, and eventually the surface-exposure of CD63, which can be readily detected by flow cytometry. Considering its features, BAT offers several advantages over prick tests and serology, incl. safety, sensitivity and specificity, a measure for severity, and consideration of blocking antibodies. However, high costs and workload, and the short time interval between blood donation and analysis (<6 h), have prevented a wider use of BAT in routine diagnostics. In addition, all known kits must be performed manually and are technically outdated, as evidenced by the high cut-off values for positivity (>15%).
The innovation provides an inexpensive, fast, user-friendly, automated high-throughput BAT with superior performance and accuracy. The flow-cytometric test exploits three specific markers, which along with a sophisticated gating strategy allow for an unambiguous discrimination between allergic, sensitized, and non-allergic individuals at lowest cut-off (2%). Sample workup and data analysis are automated, and computerized analysis generates an easy-to-understand report suitable for routine labs.
Commercial Opportunity
The innovative BAT is available for in-licensing.
Development Status
The technology has been comprehensively studied using >1,000 samples derived from allergic patients, sensitized subjects without clinical symptoms, and non-allergic individuals. Despite drastically reduced complexity, the method showed superior performance and achieved a diagnostic sensitivity and specificity of 100%. A nationwide ring trial involving ten laboratories and six different flow cytometers confirmed the method’s robustness and proved that it even tolerates the use of older blood samples stored at room temperature or 4°C for several days, dramatically increasing the operational time-window for diagnostic testing. Furthermore, the method was implemented on a flow cytometer with integrated robotic sample processing, resulting in no differences in basophil activation status compared to manually processed samples, but in time savings of more than 80%. The innovative BAT combines exceptionally specificity and sensitivity with simplified, fully automated and (largely) time-independent sample handling, processing, and analysis, qualifying it e.g., for use in central laboratories.
Patent Situation
The innovative BAT is protected by the international patent application WO2022/038217 A1.
Further Reading
Behrends J., Schwager C., Hein M., Scholzen T., Kull, S., and Jappe U. (2021) Innovative robust basophil activation test using a novel gating strategy reliably diagnosing allergy with full automation. Allergy 76: 3776-3788.
Schwager C., Kuli S., Behrends J., Röckendorf N., Schocker F., Frey A., Homann A., Becker W.-M., and Jappe U. (2017) Peanut oleosins associated with severe peanut allergy – importance of lipophilic allergens for comprehensive allergy diagnostics. Journal for Allergy and Clinical Immunology 140: 1331-1338.