Novel vaccine vector platform for safe and effective immunization using a replication-deficient murine cytomegalovirus (MCMV)
Keywords
vaccine vector, single cycle viral vector, MCMV, SARS-CoV-2, Corona, Influenza, Herpes
Invention Novelty
The invention provides a novel vaccine vector platform for safe and effective immunization against various viral pathogens using a replication-deficient murine cytomegalovirus (MCMV).
Value Proposition
An ideal vaccine offers long-term protection, causes no undesirable side effects, is safe, triggers both B-cell and T-cell responses, and needs to be administered only once. For this purpose, vaccine vectors are used to transfer genetic material of a pathogen into cells to achieve immunity through the subsequent immune response. However, previous approaches using for example adenoviral vectors have drawbacks, such as undesirable side effects and the need for multiple vaccinations.
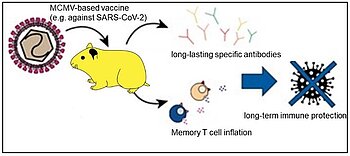
The proposed MCMV vector meets all desired requirements of a promising vaccine vector platform. CMVs belong to the herpesvirus family, characterized by specificity for the natural host species and latent virus maintenance. Sparse antigen expression during latency drives long-term protection by CMV vaccine vectors. However, the prevalence of human CMV (HCMV) in adults is approximately 70% and HCMV may cause severe disease of immunocompromised hosts or fetuses. Therefore, HCMV as a vaccine vector would be limited by natural immunity and by safety concerns. The natural immunity limitation and the adverse side effects are unlikely for the MCMV vaccine vector, because it does not replicate in human cells. Hence, MCMV acts as a safe single-cycle vector outside its cognate species. This was confirmed in proof of concept studies in hamsters, where one MCMV immunization elicited immune protection against virus challenge and B cell responses that persisted over numerous months with no warning signs, arguing that MCMVs provide lasting immunity in non-mouse species despite the lack of replication. MCMV was also tested in vitro in human cells and the observed T-cell responses were stronger than upon stimulation with HCMVs expressing the same antigen.
Technology Description
To create a viral vector platform with increased safety, the immediate early 2 gene (IE2) of murine cytomegalovirus (MCMV) was deleted. Deletion of this gene resulted in a growth deficiency of the virus in non-murine species. In contrast, replication in murine cells is unaffected, paving the way for efficient production of the vaccine. Δ-IE2-MCMV viral vectors containing full-length viral proteins from SARS-CoV-2 and Influenza A, respectively, provide long-term humoral immune protection after a single vaccination, as demonstrated in studies in hamsters.
Commercial Opportunity
The technology is available for in-licensing and co-development.
Development Status
In vivo test in hamsters using MCMV vaccines against SARS-CoV-2 showed long-term humoral immune protection.
Patent Situation
A European patent application was filed in January 2022. PCT-application was filed in January 2023.
Further Reading
Yeonsu Kim et al., MCMV-based vaccine vectors expressing full-length viral proteins provide long-term humoral immune protection upon a single-shot vaccination. Cell Mol Immunol. 2022;19 (2):234-244.